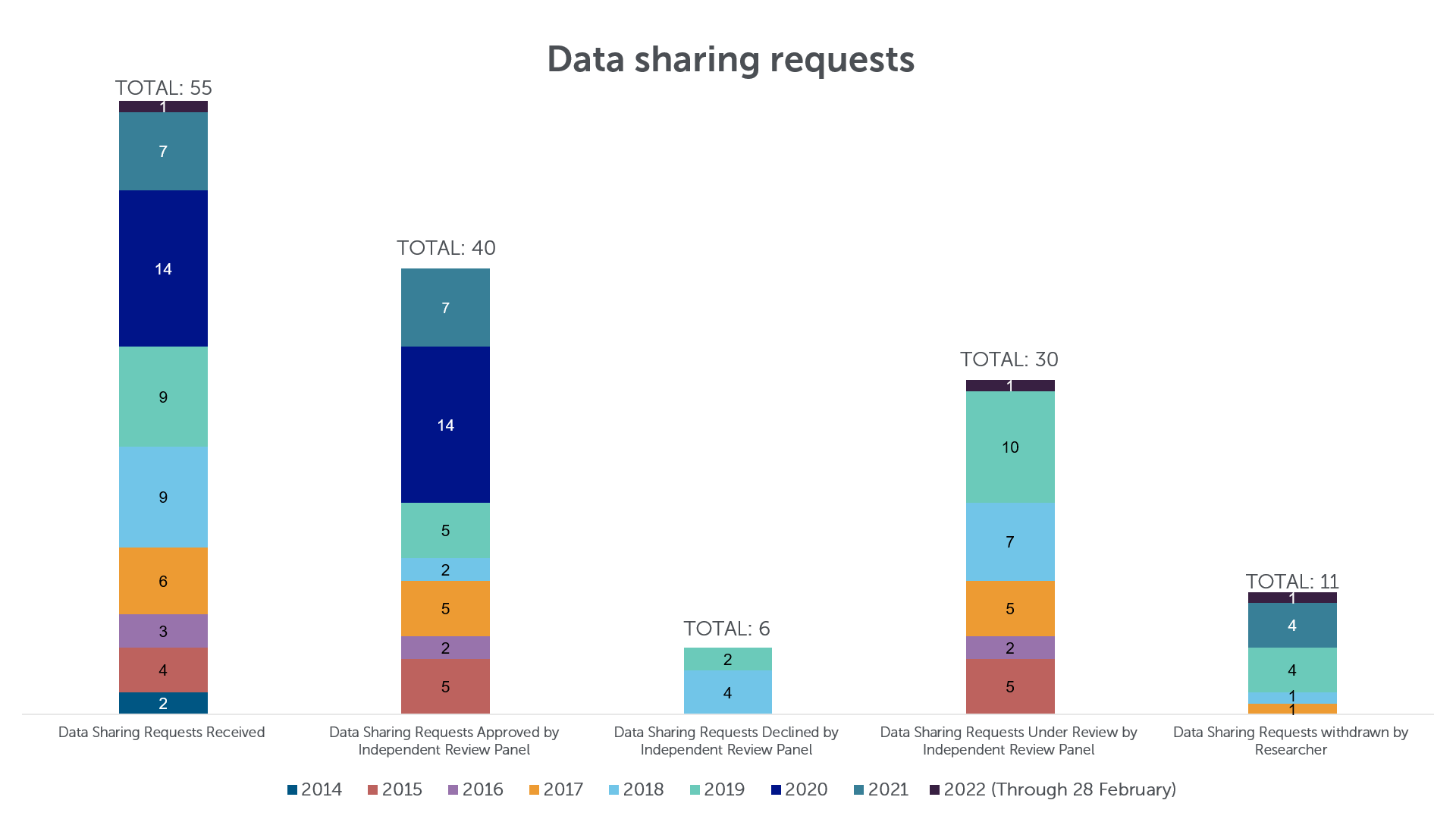
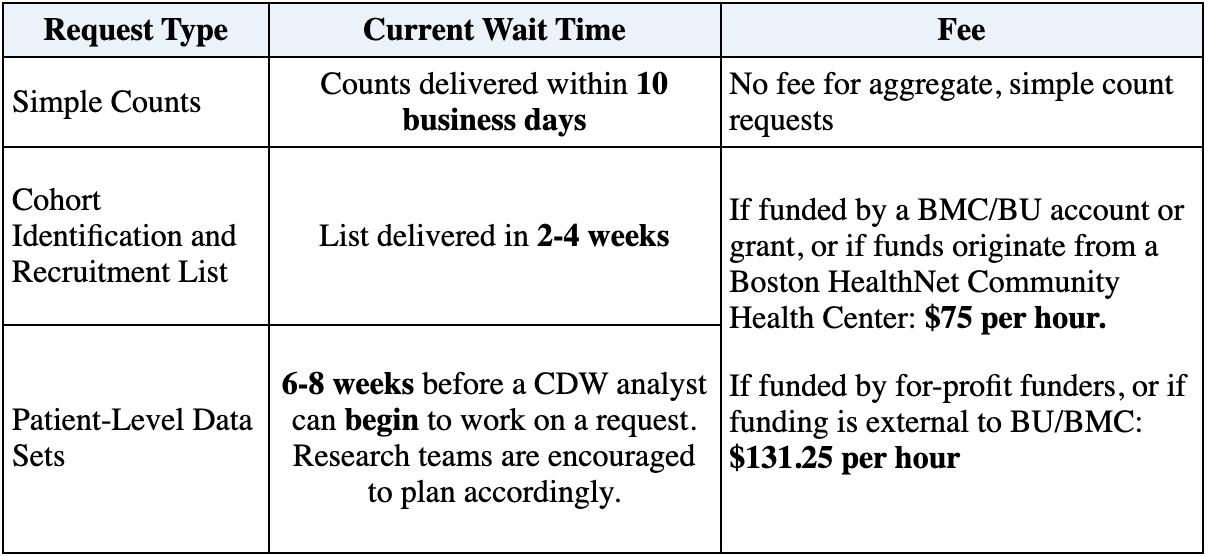
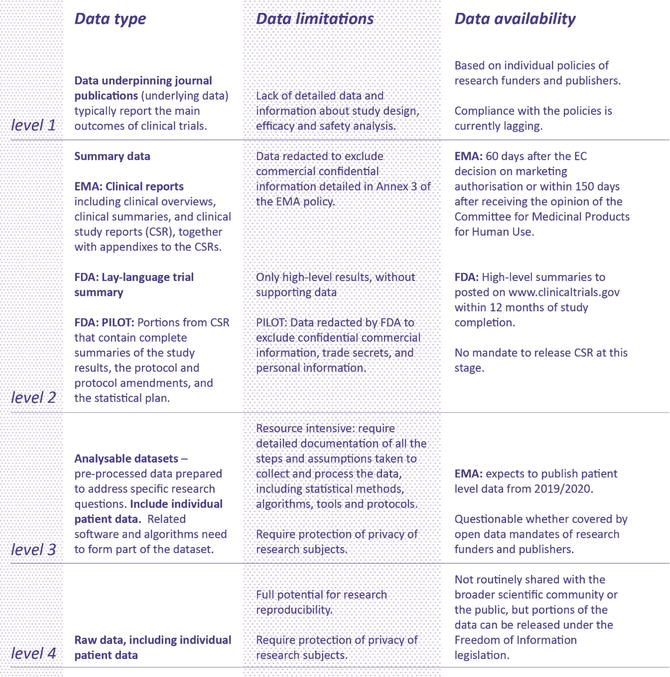
Data & Information Sharing with Qualified Researchers - Clinical Trials Data & Information Sharing - Clinical Trials - Our Science | AbbVie
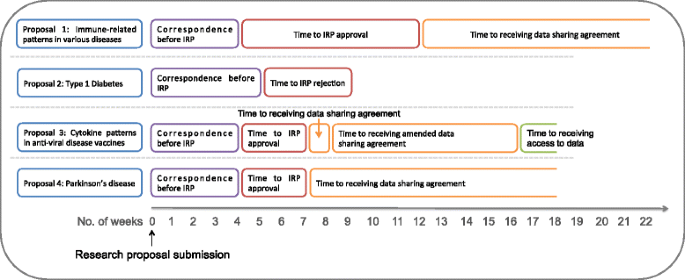
Opening clinical trial data: are the voluntary data-sharing portals enough? | BMC Medicine | Full Text
PLOS ONE: Ethics approval in applications for open-access clinical trial data: An analysis of researcher statements to clinicalstudydatarequest.com
Ethics approval in applications for open-access clinical trial data: An analysis of researcher statements to clinicalstudydatarequest.com | PLOS ONE

Individual Patient-Level Data Sharing for Continuous Learning: A Strategy for Trial Data Sharing - National Academy of Medicine

Individual Patient-Level Data Sharing for Continuous Learning: A Strategy for Trial Data Sharing - National Academy of Medicine
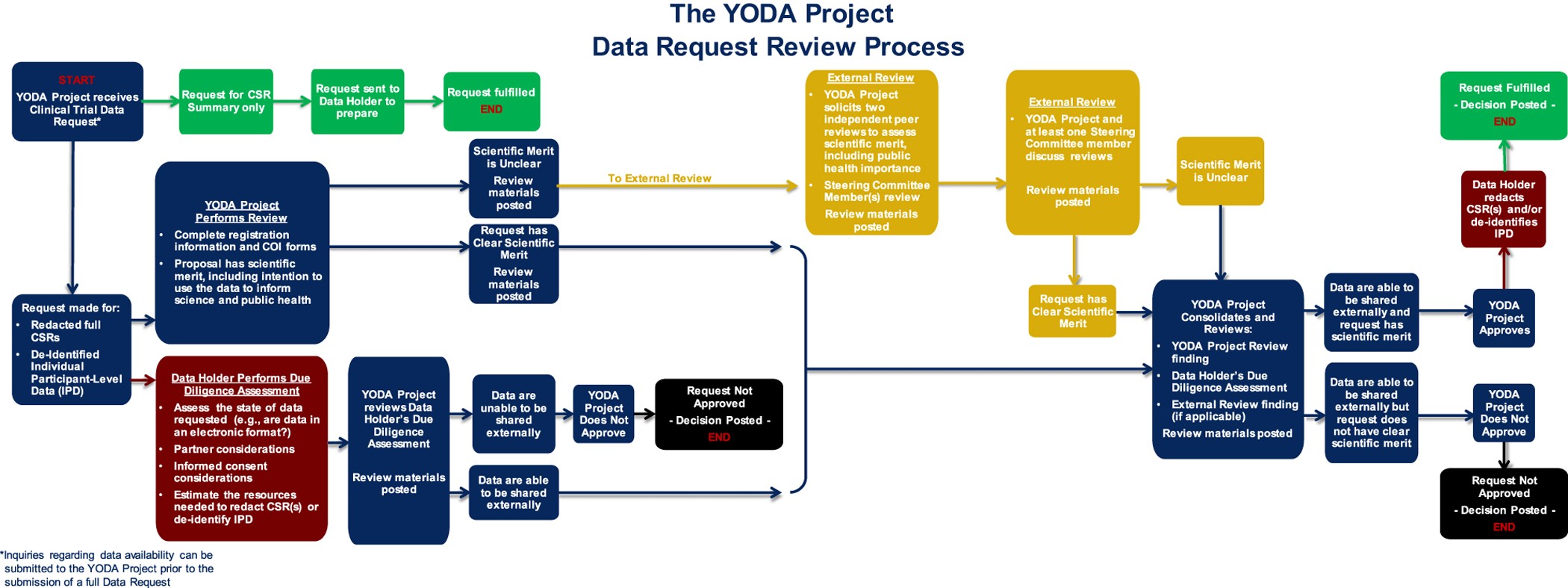
Overview and experience of the YODA Project with clinical trial data sharing after 5 years | Scientific Data

Individual Patient-Level Data Sharing for Continuous Learning: A Strategy for Trial Data Sharing - National Academy of Medicine