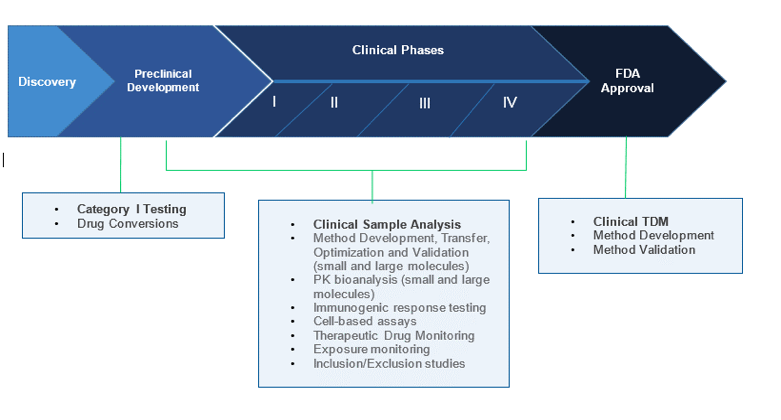
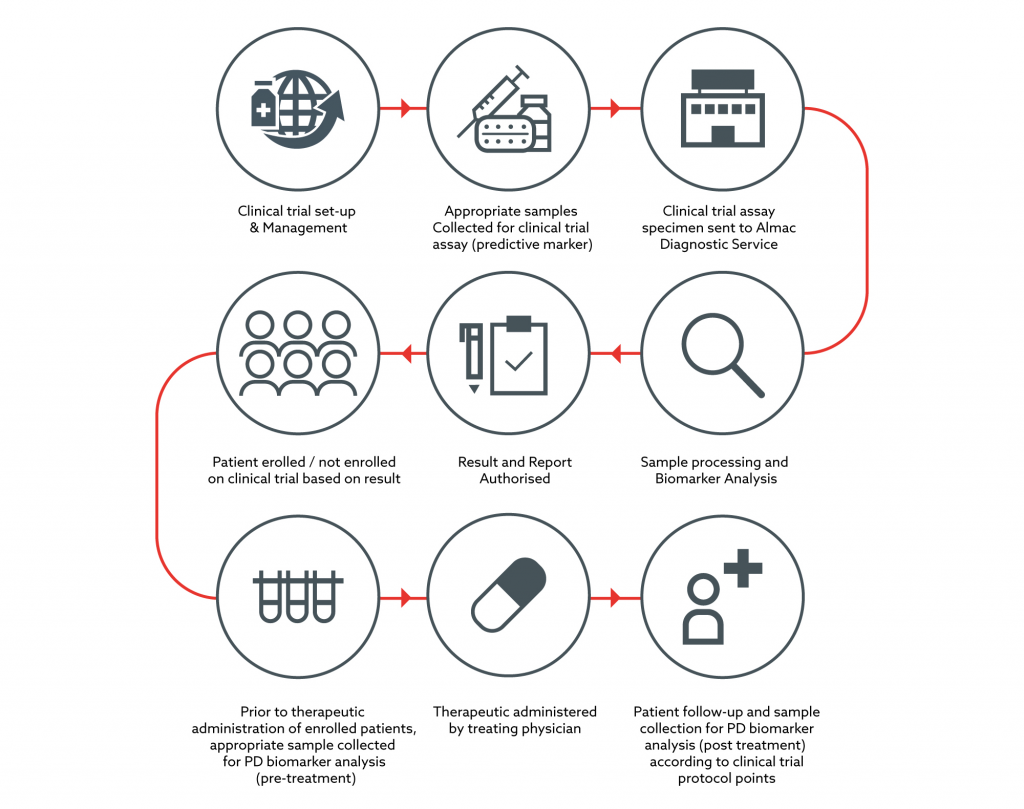
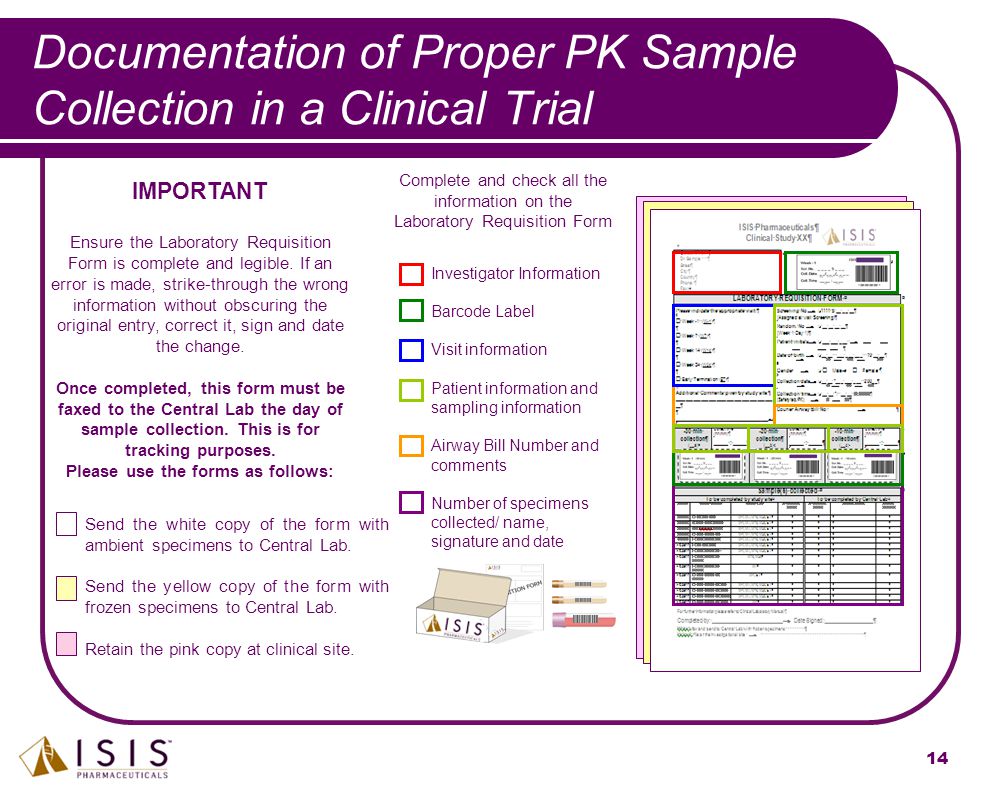
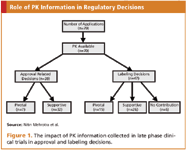
Pharmacokinetic and Statistical Considerations in First-in-Human Clinical Trials | Pharmaceutical Outsourcing - The Journal of Pharmaceutical & Biopharmaceutical Contract Services
Database of pharmacokinetic time-series data and parameters for 144 environmental chemicals | Scientific Data

Sample phase 0/2 clinical trial study design for brain tumors. Patients... | Download Scientific Diagram

Dose rationale and pharmacokinetics of dexmedetomidine in mechanically ventilated new-borns: impact of design optimisation | SpringerLink

Guidelines for the experimental design of pharmacokinetic studies with nanomaterials in preclinical animal models - ScienceDirect

Pharmaceutics | Free Full-Text | Pharmacokinetic-Pharmacodynamic Modelling of Systemic IL13 Blockade by Monoclonal Antibody Therapy: A Free Assay Disguised as Total | HTML

PDF) Population pharmacokinetic analysis for risperidone using highly sparse sampling measurements from the CATIE study | Margaret Kirshner - Academia.edu

Phase 0 clinical trials in oncology new drug development Gupta UC, Bhatia S, Garg A, Sharma A, Choudhary V - Perspect Clin Res
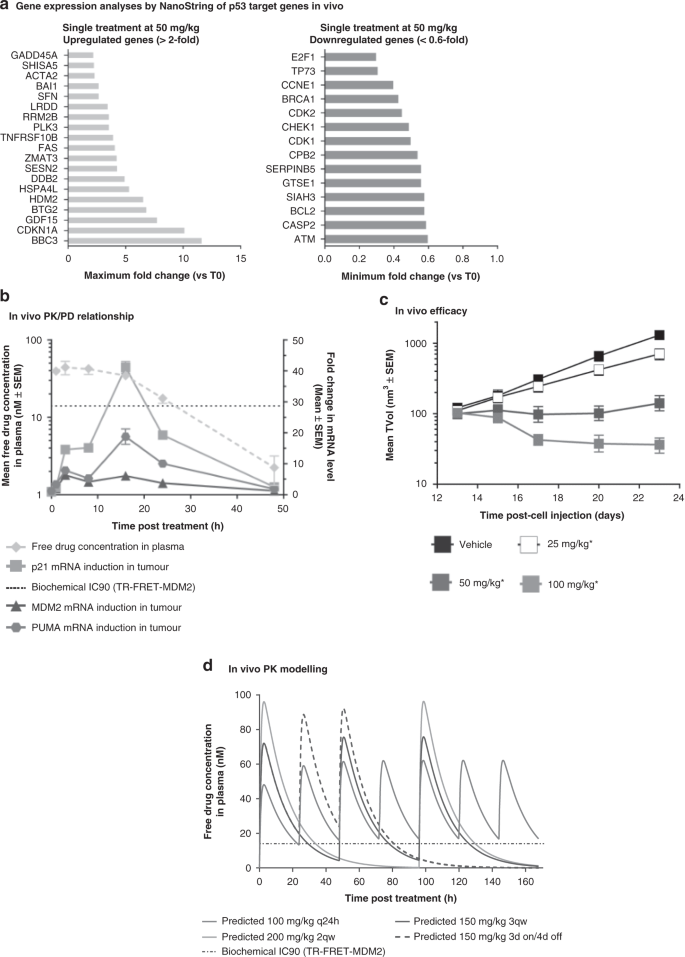
Pharmacokinetic–pharmacodynamic guided optimisation of dose and schedule of CGM097, an HDM2 inhibitor, in preclinical and clinical studies | British Journal of Cancer

The importance of clinical pharmacokinetic–pharmacodynamic studies in unraveling the determinants of early and late tuberculosis outcomes | International Journal of Pharmacokinetics

Simplified dolutegravir dosing for children with HIV weighing 20 kg or more: pharmacokinetic and safety substudies of the multicentre, randomised ODYSSEY trial - The Lancet HIV

Improving the Accuracy of Predicted Human Pharmacokinetics: Lessons Learned from the AstraZeneca Drug Pipeline Over Two Decades: Trends in Pharmacological Sciences

Phase 1 clinical trial schema. Abbreviations: E = enrollment, qwk = per... | Download Scientific Diagram

Pharmacokinetic and Statistical Considerations in First-in-Human Clinical Trials | Pharmaceutical Outsourcing - The Journal of Pharmaceutical & Biopharmaceutical Contract Services